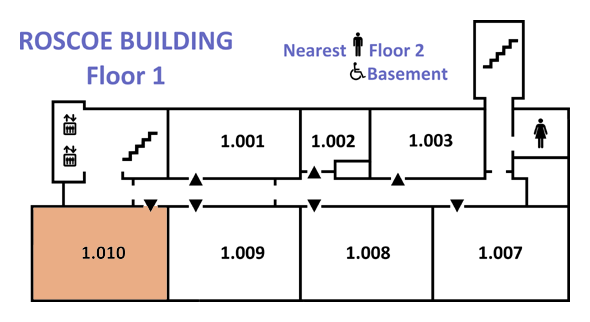
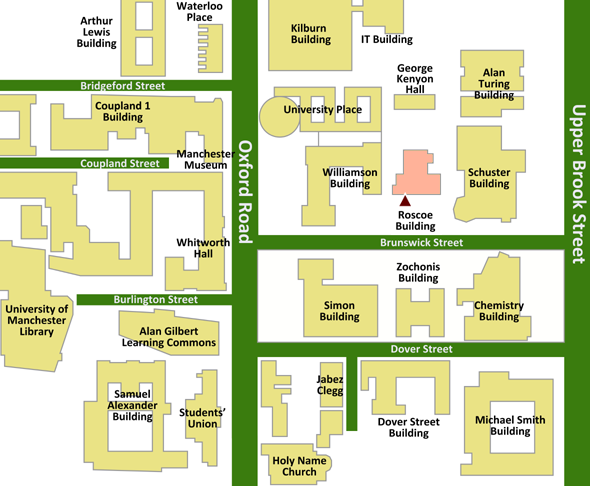
|
iCHSTM 2013 Programme • Version 5.3.6, 27 July 2013 • ONLINE (includes late changes)
Index | Paper sessions timetable | Lunch and evening timetable | Main site |

|
iCHSTM 2013 Programme • Version 5.3.6, 27 July 2013 • ONLINE (includes late changes)
Index | Paper sessions timetable | Lunch and evening timetable | Main site |
It has become almost commonplace since the 19th century to emphasize how much chemists shape matter and build new materials, not only to enhance natural knowledge, but also in the hope of improving the human condition. By creating new, hopefully useful substances, chemists have established a role, not only in science and technology but also as architects of both matter and society. Less often stressed is how materials may in turn shape chemists and their science, both by creating or reorganizing disciplinary fields, communities, instrumental consensus and experimental practice and objects, and by initiating new behaviours in society and consumption or adding to the ever growing number of synthetics.
For example, consider the solid compounds extensively synthesized by inorganic chemists in the twentieth century. This led in the 1960s to the emergence of a new subdiscipline: solid state chemistry. Referring to themselves as “solidists”, solid state chemists became identified by their will to synthesize original solid compounds. These were shaped at the inner (atomic) level to exhibit new intrinsic properties. These pieces of matter rapidly escaped their creators to be transformed into “materials” by a new field, materials chemists, working at an intermediary (microscopic) level to turn solid compounds into commercial products with many uses, including ceramics, metallurgy, and electronics. The step from matter to materials was induced by the transformation of a bench compound to a brand product when solid state chemists, who exhibited properties, were replaced by materials chemists who stabilized the compound to make it useful. In the process two new subdisciplines of chemistry emerged. Ultimately the resulting new products have had a significant impact on modern consumption patterns and material culture. Introducing new materials into the environment has also posed unexpected challenges for regulation, clean-up, and recycling, which have in turn affected the activities of chemists and have led to the emergence of another new field, “green chemistry.”
There is thus a co-construction of the subject and object of chemistry, and in the frame of this symposium, we would like to invite further considerations of the mirror dynamism between people and materials in a wide range of interacting fields and levels of activity from bench research through engineering to human society and the natural environment. Up to now this has been mostly underlined in the field of inert or non-living materials, as in our example (other examples from the twentieth century could include the development of synthetic polymers and plastics); we also welcome case-studies and considerations from the life sciences, where the creation of new drugs or food ingredients may follow a similar trajectory (or not).